By Tom Mansell, Science Editor
In my last article on ice wine, I talked about how volatile acidity is an important aroma characteristic in ice wine and how it can contribute to peach and pineapple aromas. To conclude our discussion, let's talk about some other famous ice wine descriptors, including honey.
 For our purposes, it's best to think of ice wine grapes as late harvest (well, really late harvest). Grapes are left to hang until December, January, and even February until they are frozen on the vine. During this time, all kinds of physical, chemical, and biological changes are happening to the berries.
For our purposes, it's best to think of ice wine grapes as late harvest (well, really late harvest). Grapes are left to hang until December, January, and even February until they are frozen on the vine. During this time, all kinds of physical, chemical, and biological changes are happening to the berries.
First, as the season draws to a close, the berries will continue to accumulate sugar (until photosynthesis stops) and lose malic acid to respiration. This loss of acid can have a huge effect on the finished wine, which may even require acid addition to balance the huge residual sugar.
Cool-climate winemakers may find it absurd to have to add acid (and may feel slightly dirty doing so), but in this case the sweetness of the finished wine may demand it.
Second, noble rot (Botrytis cinerea) may start to move in, especially in tight-clustered varieties like riesling and vidal blanc. This mold penetrates the skin of the grape, drying out the berries and concentrating flavors, sugars, and acids.
When berries are carefully selected, botrytis can be responsible for heavenly Sauternes and Trockenbeerenauslese-style wines.
Third, the skins of the berries can be physically damaged by birds, deer or simply freezing. Again, this can lead to berries drying out, but it can also lead to growth of other less-desirable organisms. The overall loss due to grape damage and deer breakfast partially justifies the higher-than-normal price of ice wine.
Aroma compounds
The combination of these three factors can lead to the formation of aroma compounds not usually found in dry wines. Over-ripening has been found to lead to development of compounds normally found in older and/or oxidized wines. While the aroma profile of ice wines has not been studied extensively, I think it's safe to speculate that the damage to berries in ice wine shares characteristics with botrytized berries, and many botrytized wine aroma compounds have been recently characterized. They include:
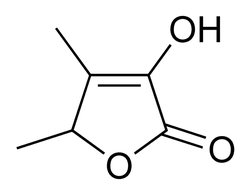 Sotolon (spicy, curry, honey) (pictured at right)
Sotolon (spicy, curry, honey) (pictured at right)- Furaneol (strawberry, cotton candy)
- Phenylacetaldehyde (honey, rose)
While biosynthesis of these compounds is not fully understood, it's likely that the first two are the products of sugar oxidation either in the berries themselves or by yeast during fermentation. Phenylacetaldehyde is produced by the botrytis itself, and is also a key aroma component in honey.
Mouthfeel
As if smelling like honey weren't enough to provide the "honey" descriptor, ice wine's dense and rich mouthfeel make it feel like honey on the tongue. This increased perceived viscosity is not due to glycerol formation (although glycerol in ice wine is higher than in dry wines). At the levels present in wine, glycerol does not contribute to increased perceived density or viscosity.
The real culprit of increased density and viscosity is high residual sugar. This shouldn't be too surprising to anyone who knows that measurement of Brix, i.e., the concentration of sugars in a must, is based on density.
Overall, with its concentrated aroma profile featuring fruity and botrytized aromas (usually with a smidge of VA), and dense mouthfeel, ice wine is really something special. It's something I'd like to see more of in the Finger Lakes. No doubt that it gets cold enough. When conditions are right and harvest and fermentation are well controlled by vineyard management and winemaker, the results can be outstanding.
Further Reading:
Nurgel and Pickering, "Contribution of glycerol, ethanol and sugar to the perception of viscosity and density elicited by model white wines." Journal of Texture Studies, 2005
Sarrazin et al., "Characterization of key-aroma compounds of botrytized wines, influence of grape botrytization." Food Chemistry, 2007.
To assist with this work, I tasted Sheldrake Point's 2007 Riesling Ice Wine and 2008 Cabernet Franc Ice Wine. For my tasting notes, see my writeup on Ithacork.
Thanks to Sheldrake Point, especially Dave Breeden for helpful insights.
